Abstract
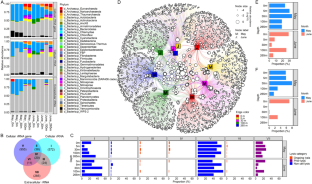
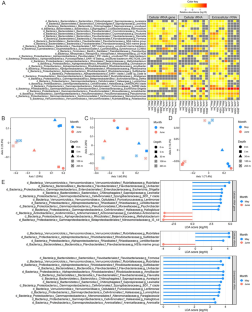
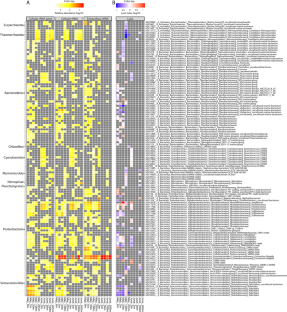
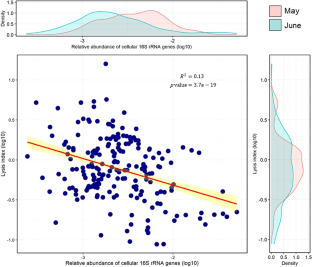
Microbes are by far the dominant biomass in the world’s oceans and drive biogeochemical cycles that are critical to life on Earth. The composition of marine microbial communities is highly dynamic, spatially and temporally, with consequent effects on their functional roles. In part, these changes in composition result from viral lysis, which is taxon-specific and estimated to account for about half of marine microbial mortality. Here, we show that extracellular ribosomal RNA (rRNAext) is produced by viral lysis, and that specific lysed populations can be identified by sequencing rRNAext recovered from seawater samples. In ten seawater samples collected at five depths between the surface and 265 m during and following a phytoplankton bloom, lysis was detected in about 15% of 16,946 prokaryotic taxa, identified from amplicon sequence variants (ASVs), with lysis occurring in up to 34% of taxa within a water sample. The ratio of rRNAext to cellular rRNA (rRNAcell) was used as an index of taxon-specific lysis, and revealed that higher relative lysis was most commonly associated with copiotrophic bacteria that were in relatively low abundance, such as those in the genera Escherichia and Shigella spp., as well as members of the Bacteriodetes; whereas, relatively low lysis was more common in taxa that are often relatively abundant, such as members of the Pelagibacterales (i.e., SAR11 clade), cyanobacteria in the genus Synechococcus, and members of the phylum Thaumarchaeota (synonym, Nitrososphaerota) that comprised about 13–15% of the 16 S rRNA gene sequences below 30 m. These results provide an explanation for the long-standing conundrum of why highly productive bacteria that are readily isolated from seawater are often in very low abundance. The ability to estimate taxon-specific cell lysis will help explore the distribution and abundance of microbial populations in nature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to Journal
Get full journal access for 1 year
118,99 €
only 9,92 € per issue
Tax calculation will be finalised during checkout.
Buy article
Get time limited or full article access on ReadCube.
$32.00
All prices are NET prices.


Data availability
Sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive (SRA) under the accession numbers SRR14873150 to SRR14873179.
Code availability
The related codes for analyzing the taxon-specific lysis (lysis-index and lysis-rate) are included in the custom R package: tslysis (https://github.com/kevinzhongxu/tslysis).
References
Fuhrman JA, Cram JA, Needham DM. Marine microbial community dynamics and their ecological interpretation. Nat Rev Microbiol. 2015;13:133–46.
Suttle CA. Viruses in the sea. Nature 2005;437:356–61.
Wilhelm SW, Suttle CA. Viruses and nutrient cycles in the sea: Viruses play critical roles in the structure and function of aquatic food webs. BioScience 1999;49:781–8.
Fuhrman JA. Marine viruses and their biogeochemical and ecological effects. Nature 1999;399:541–8.
Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol Rev. 2004;28:127–81.
Bayles KW. Bacterial programmed cell death: making sense of a paradox. Nat Rev Microbiol. 2014;12:63–69.
Williams HN, Lymperopoulou DS, Athar R, Chauhan A, Dickerson TL, Chen H, et al. Halobacteriovorax, an underestimated predator on bacteria: potential impact relative to viruses on bacterial mortality. ISME J. 2016;10:491–9.
Pérez J, Moraleda-Muñoz A, Marcos-Torres FJ, Muñoz-Dorado J. Bacterial predation: 75 years and counting! Environ Microbiol. 2016;18:766–79.
Thingstad TF. Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol Oceanogr. 2000;45:1320–8.
Breitbart M, Rohwer F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005;13:278–84.
Suttle CA. Marine viruses–major players in the global ecosystem. Nat Rev Microbiol. 2007;5:801–12.
Suttle CA, Chen F. Mechanisms and rates of decay of marine viruses in seawater. Appl Environ Microbiol. 1992;58:3721–9.
Dai W, Fu C, Raytcheva D, Flanagan J, Khant HA, Liu X, et al. Visualizing virus assembly intermediates inside marine cyanobacteria. Nature 2013;502:707–10.
Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17:13–24.
González J, Suttle CA. Grazing by marine nanoflagellates on viruses and virus-sized particles: ingestion and digestion. Mar Ecol Prog Ser. 1993;94:1–10.
Seong KA, Jeong HJ, Kim S, Kim GH, Kang JH. Bacterivory by co-occurring red-tide algae, heterotrophic nanoflagellates, and ciliates. Mar Ecol Prog Ser. 2006;322:85–97.
Sherr BF, Sherr EB, Fallon RD. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987;53:958–65.
Datta AK, Burma DP. Association of ribonuclease I with ribosomes and their subunits. J Biol Chem. 1972;247:6795–801.
Deutscher MP. Maturation and degradation of ribosomal RNA in bacteria. Prog Mol Biol Transl Sci. 2009;85:369–91.
Timko SA, Maydanov A, Pittelli SL, Conte MH, Cooper WJ, Koch BP, et al. Depth-dependent photodegradation of marine dissolved organic matter. Front Mar Sci. 2015;66:1–13.
Noble RT, Fuhrman JA. Virus decay and its causes in coastal waters. Appl Environ Microbiol. 1997;63:77–83.
Wilhelm SW, Jeffrey WH, Suttle CA, Mitchell DL. Estimation of biologically damaging UV levels in marine surface waters with DNA and viral dosimeters. Photochem Photobio. 2002;76:268–73.
Wurtmann EJ, Wolin SL. RNA under attack: cellular handling of RNA damage. Crit Rev Biochem Mol Biol. 2009;44:34–49.
Paul JH, Jeffrey WH, DeFlaun MF. Dynamics of extracellular DNA in the marine environment. Appl Environ Microbiol. 1987;53:170–9.
Bong CW, Obayashi Y, Suzuki S. Succession of protease activity in seawater and bacterial isolates during starvation in a mesocosm experiment. Aquat Micro Ecol. 2013;69:33–46.
Breitbart M, Bonnain C, Malki K, Sawaya NA. Phage puppet masters of the marine microbial realm. Nat Microbiol. 2018;3:754–66.
Blazewicz SJ, Barnard RL, Daly RA, Firestone MK. Evaluating rRNA as an indicator of microbial activity in environmental communities: limitations and uses. ISME J. 2013;7:2061–8.
Fuhrman JA, Noble RT. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol Oceanogr. 1995;40:1236–42.
Mojica KDA, Brussaard CPD. Significance of viral activity for regulating heterotrophic prokaryote community dynamics along a meridional gradient of stratification in the Northeast Atlantic Ocean. Viruses 2020;12:1293.
Suttle CA. The significance of viruses to mortality in aquatic microbial communities. Micro Ecol. 1994;28:237–43.
Bibby K. Improved bacteriophage genome data is necessary for integrating viral and bacterial ecology. Micro Ecol. 2014;67:242–4.
Labonté JM, Swan BK, Poulos B, Luo H, Koren S, Hallam SJ, et al. Single-cell genomics-based analysis of virus–host interactions in marine surface bacterioplankton. ISME J. 2015;9:2386–99.
Sockett RE. Predatory lifestyle of Bdellovibrio bacteriovorus. Annu Rev Microbiol. 2009;63:523–39.
Chao L, Levin BR. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc Natl Acad Sci USA. 1981;78:6324–8.
Granato ET, Meiller-Legrand TA, Foster KR. The evolution and ecology of bacterial warfare. Curr Biol. 2019;29:R521–R537.
Bremer H, Dennis PP Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt FC, Ingraham JL, Magasanik B, Low KB, Schaechter M, Umbarger HE (eds). Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. 2nd ed. American Society for Microbiology, 1996. pp. 1559, Tab. 3.
Landa M, Cottrell MT, Kirchman DL, Blain S, Obernosterer I. Changes in bacterial diversity in response to dissolved organic matter supply in a continuous culture experiment. Aquat Micro Ecol. 2013;69:157–68.
Waterbury JB, Valois FW. Resistance to co-occurring phages enables marine synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol. 1993;59:3393–9.
Sullivan MB, Waterbury JW, Chisholm SW. Cyanophages infecting the oceanic cyanobacterium Prochlorococcus. Nature 2003;424:1047–51.
Våge S, Storesund JE, Thingstad TF. SAR11 viruses and defensive host strains. Nature 2013;499:E3–4.
Bouvier T, del Giorgio PA. Key role of selective viral-induced mortality in determining marine bacterial community composition. Environ Microbiol. 2007;9:287–97.
Stahl DA, de la Torre JR. Physiology and diversity of ammonia-oxidizing Archaea. Annu Rev Microbiol. 2012;66:83–101.
Zhao Y, Temperton B, Thrash JC, Schwalbach MS, Vergin KL, Landry ZC, et al. Abundant SAR11 viruses in the ocean. Nature 2013;494:357–60.
Kerkhof L, Kemp P. Small ribosomal RNA content in marine Proteobacteria during non-steady-state growth. FEMS Microbiol Ecol. 1999;30:253–60.
Campbell BJ, Yu L, Heidelberg JF, Kirchman DL. Activity of abundant and rare bacteria in a coastal ocean. Proc Natl Acad Sci USA. 2011;108:12776–81.
Alves MS, Pereira A, Araújo SM, Castro BB, Correia ACM, Henriques I. Seawater is a reservoir of multi-resistant Escherichia coli, including strains hosting plasmid-mediated quinolones resistance and extended-spectrum beta-lactamases genes. Front Microbiol. 2014;5:426.
Cohen R, Paikin S, Rokney A, Rubin-Blum M, Astrahan P. Multidrug-resistant enterobacteriaceae in coastal water: an emerging threat. Antimicrob Resist Infect Control. 2020;9:169.
Gorrasi S, Pasqualetti M, Franzetti A, González-Martínez A, González-López J, Muñoz-Palazon B, et al. Persistence of Enterobacteriaceae Drawn into a Marine Saltern (Saline di Tarquinia, Italy) from the Adjacent Coastal Zone. Water 2021;13:1443.
Sunagawa S, Coelho LP, Chaffron S, Kultima JR, Labadie K, Salazar G, et al. Structure and function of the global ocean microbiome. Science 2015;348:1261359.
Fernandes V, Bogati K. Persistence of fecal indicator bacteria associated with zooplankton in a tropical estuary-west coast of India. Environ Monit Assess. 2019;191:420.
Rocchini RJ, Bergerud WA, Drinna RW. FRASER RIVER ESTUARY STUDY,. WATER QUALITY,. SURVEY OF FECAL COLIFORMS IN 1978. APD Bulletin 21, Province of British Columbia, Ministry of Environment, Assessment and Planning Division. 1981.
Guillard RRL. Culture of Phytoplankton for Feeding Marine Invertebrates. In: Smith WL, Chanley MH (eds). Culture of Marine Invertebrate Animals: Proceedings - 1st Conference on Culture of Marine Invertebrate Animals Greenport. Springer US, 1975. pp. 29-60.
Caron DA Enrichment, Isolation, and Culture of Free-Living Heterotrophic Flagellates. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds). Handbook of Methods in Aquatic Microbial Ecology. CRC Press, 1993. pp. 77–89.
Mioni C, Poorvin L, Wilhelm S. Virus and siderophore-mediated transfer of available Fe between heterotrophic bacteria: characterization using an Fe-specific bioreporter. Aquat Micro Ecol. 2005;41:233–45.
Hennes KP, Suttle CA, Chan AM. Fluorescently labeled virus probes show that natural virus populations can control the structure of marine microbial communities. Appl Environ Microbiol. 1995;61:3623–7.
Abedon ST. Lysis from without. Bacteriophage 2011;1:46–49.
Marie D, Partensky F, Vaulot D, Brussaard CPD. Enumeration of phytoplankton, bacteria, and viruses in marine samples. Curr Protoc Cytom. 2001;Chapter 11:Unit 11.11.
Brussaard CPD. Optimization of procedures for counting viruses by flow cytometry. Appl Environ Microbiol. 2004;70:1506–13.
Rose JM, Caron DA, Sieracki ME, Poulton N. Counting heterotrophic nanoplanktonic protists in cultures and aquatic communities by flow cytometry. Aquat Micro Ecol. 2004;34:263–77.
Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiol Read Engl. 2002;148:257–66.
Sekiguchi H, Watanabe M, Nakahara T, Xu B, Uchiyama H. Succession of bacterial community structure along the changjiang river determined by denaturing gradient gel electrophoresis and clone library analysis. Appl Environ Microbiol. 2002;68:5142–50.
Anonymous. 16S Metagenomic Sequencing Library Preparation. Illumina Inc. 2013. Available at https://support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html.
Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016;18:1403–14.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014;30:2114–20.
Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina Paired-End read merger. Bioinformatics 2014;30:614–20.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP, et al. DADA2: High resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–596.
McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLOS ONE. 2013;8:e61217.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, 2020. https://www.R-project.org/.
Wickham H ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York, 2016. https://ggplot2.tidyverse.org.
Csardi G, Nepusz T The igraph software package for complex network research. InterJournal, Complex Systems 1695, 1-9 (2006).
Aphalo PJ Learn R…as you learnt your mother tongue. Leanpub, Helsinki, 2017. https://leanpub.com/learnr.
Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–35.
Anderson MJ Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online. 2017. American Cancer Society, pp 1–15.
Oksanen FJ, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, et al. Vegan: Community Ecology Package. R package Version 2.5-7. 2020. URL: https://CRAN.R-project.org/package=vegan
Lahti L, Shetty S Tools for microbiome analysis in R. Microbiome package version 1.13.12. 2017. URL: http://microbiome.github.com/microbiome
Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods. 2012;9:811–4.
Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018;46:W537–W544.
Acknowledgements
We thank members of the Hakai Institute for facilitating the collection of seawater samples, particularly Brian Hunt, Kate Lansley, Alex Hare and Megan Foss. We are grateful to David Caron for providing Paraphysiomonas bandaiensis for the grazing studies. Comments from the editor and two anonymous reviewers are gratefully acknowledged and were instrumental in improving the manuscript. This work was supported by grants to CAS from the Tula Foundation, the Gordon and Betty Moore Foundation (grant: GBMF#5600), a Discovery grant from the Natural Sciences and Engineering Research Council of Canada, and infrastructure awards from the Canada Foundation for Innovation and the British Columbia Knowledge Development Fund.
Author information
Authors and Affiliations
Contributions
KXZ designed experimental approaches, conducted experimental work, analyzed the data, wrote the initial draft of the manuscript, and oversaw subsequent versions. JFW conducted initial experimental work and edited the manuscript. AMC provided technical support throughout the project and edited the manuscript. CAS conceived the project, contributed to experimental design and data interpretation, and helped write the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, K.X., Wirth, J.F., Chan, A.M. et al. Mortality by ribosomal sequencing (MoRS) provides a window into taxon-specific cell lysis. ISME J (2022). https://doi.org/10.1038/s41396-022-01327-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41396-022-01327-3
https://news.google.com/__i/rss/rd/articles/CBMiMmh0dHBzOi8vd3d3Lm5hdHVyZS5jb20vYXJ0aWNsZXMvczQxMzk2LTAyMi0wMTMyNy0z0gEA?oc=5
2022-10-08 13:22:05Z
CBMiMmh0dHBzOi8vd3d3Lm5hdHVyZS5jb20vYXJ0aWNsZXMvczQxMzk2LTAyMi0wMTMyNy0z0gEA
Tidak ada komentar:
Posting Komentar