Initially, 30 CAM journals were retrieved from JCR (2021). After reviewing the aims and scope of each journal, PLANTA MEDICA and Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas were excluded because the acceptance scopes of these 2 journals did not include biomedical research involving humans, BMC Complementary and Alternative Medicine was excluded from Q1 section due to the change to BMC Complementary Medicine and Therapies, finally, 27 journals remained.
General information about included journals
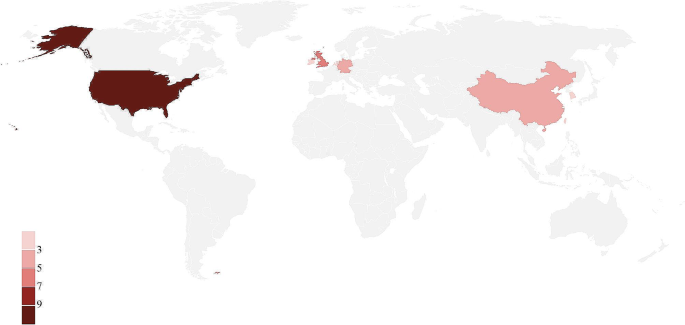
The 27 journals originated in eight different nations and areas (Table 1). Ten journals were from the United States, five from England, four from China’s mainland, three from Germany, two from South Korea, and one each from Ireland, the Netherlands, and Taiwan, China (Fig. 2). 27 journals were published by 12 different publishers.
The requirements for ethical review in IFAs
Of the 27 journals included in the study, 92.6% (25/27) IFAs contained keywords of “ethic(s)”, “ethical”, or “human” in the subtitles and text words, which represented there were ethical considerations in these journals. Of these, 84.0% (21/25) explicitly mentioned that the manuscript of biomedical research involving human subjects should undergo ethical review; 12.0% (3/25) IFAs’ (Journal of Integrative Medicine, Journal of Herbal Medicine, ACUPUNCTURE & ELECTRO-THERAPEUTICS RESEARCH) content of ethical review regarding the policy of publishing ethics most and authors needed to read IFAs carefully to search for key information; 4.0% (1/25) only mentioned ethics in publication (Holistic Nursing Practice). 7.4% (2/27) IFAs of journals (Phytomedicine, Chinese Journal of Integrative Medicine) had no clear claim to ethical review of medical research involving human subjects in the subtitles or text words of their IFAs, but there was an “ethics disclosures” on the official website page of Chinese Journal of Integrative Medicine, IFA of Phytomedicine declared that “the author should ensure that the manuscript contains a statement that all procedures were performed in compliance with relevant laws and institutional guidelines and that the appropriate institutional committee(s) have approved them”.
Citation situation of the DoH, ICMJE recommendations, and COPE Core Practice
In this study, 81.5% (22/27) of the IFAs mentioned the Declaration of Helsinki (DoH), 70.4% (19/27) of the IFAs mentioned ICMJE recommendations. 21 journals are members of COPE, although 3 journals had not yet become members of COPE, their IFAs also required authors to follow COPE core practice.
In addition to the above international general guidelines for ethical review, the IFAs of Phytomedicine recommended that authors comply with ICH-E6 Good Clinical Practice [17]. Policy Statement on Geopolitical Intrusion on Editorial Decisions issued by the World Association of Medical Editors (WAME), UK’s The Medicines for Human Use (Clinical Trials) Regulations, ICMJE Recommendations for the Protection of Research Participants, NLM’s Research Reporting Guidelines, and Initiatives and Guidelines for the Conduct of Human Embryonic Stem Cell Research established by the International Society for Stem Cell Research (ISSCR) always appeared in IFAs.
The results showed there was no statistical significance in the citation of the DoH, ICMJE recommendations and COPE core practice in any classification of journals (p > 0.05, Table 2). For the factors related to journals that we have taken into account above, they were not influencing factors for CAM journals to make a particular ethical review request. We integrated journals that cited the above three documents (the DoH, ICMJE recommendations, COPE guidelines) at the same time, and calculated their mean value IF: 3.20 (3.05), mean value years of electronic JCR: 8.8 (14.9), median % OA GOLD: 14.97% (5.42%), and the data of journals that do not cite the above three documents are in parentheses.
The requirements of IRB approval, name of IRB, IRB approval number, registration and reporting guidelines
Many journals also requested informations on IRB, but Geographic origin, JCR section, Year of electronic JCR, Types of studies, % of OA Gold were not associated with requirements of IRB approval, name of IRB, IRB approval number, registration and reporting guidelines separately (p > 0.05, Table 2). Some journals required that the authors provide the details (JOURNAL OF ALTERNATIVE AND COMPLEMENTARY MEDICINE) of the ethical review process and the date of ethical review (American Journal of Chinese Medicine) along with manuscripts.
Policies of IC, images privacy and data sharing
In addition to adhering to the international guidelines mentioned above, some journals emphasized the principles of IC and patient privacy. 81.5% (22/27) of journals mentioned obtaining IC from patients, some of the journals (Journal of Traditional and Complementary Medicine, COMPLEMENTARY THERAPIES IN MEDICINE, INTEGRATIVE CANCER THERAPIES) required that patients’ handwritten IC be retained and backed up, some of the journals (Complementary Medicine Research, JOURNAL OF MANIPULATIVE AND PHYSIOLOGICAL THERAPEUTICS) required authors to provide a statement of detailed procedure in obtaining IC. Among these journals, 50.0% (11/22) of them proposed protecting patient privacy as well. As for the use of patients’ images and photographs, 51.9% (14/27) of the journals emphasized the need to obtain IC from patients before using their photos, and that some identifying information should be hidden, but there were no more separate and specific consent was required. 77.8% (21/27) of journals promoted data sharing and make research more transparent, these journals encouraged authors to share their research data, which refers to the “results of observations or experimentation that validate research findings”, but there were no policies relevant for personal data protection.
We integrated journals which had a requirement of IRB approval, name of IRB, IRB approval number, registration, reporting guidelines along with IC, and calculated their mean value IF: 2.96 (3.19), mean value years of electronic JCR: 6.7 (13.7), median % OA GOLD: 98.71% (7.03%), and the data of journals that do not have the requirements above are in parentheses.
It seems that CAM journals which were included in electronic JCR in recent years, and the higher the % OA GOLD, will have more comprehensive requirements for ethical review.
The actual situation of ethical requirement in published manuscripts
We also browsed the manuscripts regarding randomized controlled trials (RCT) published by CAM journals in Q1 and Q2 section from January to June, 2023, to check the actual situation of ethical requirement. There were 68 manuscripts (20 from Q1 section, 48 from Q2 section) in total (Table 3). Of the 20 randomized controlled studies included in Q1, 11 studies were from China, 4 from Korea, 2 from Iran, and 1 from Brazil, Australia, and the United States, respectively. 95.00% (19/20) manuscripts mentioned that their research had been registered on the website, 90.00% (18/20) of which also gave registration numbers, and only one [18] did not mention any registration information about the clinical trial. Of the 48 randomized controlled studies included in Q2, 95.83% (46/48) manuscripts mentioned that their research had been registered on the website, 91.67% (44/48) of which gave registration numbers, 4.17% (2/48) manuscripts [19, 20] did not contain the information about trial registration. In Q1 section, all the manuscripts mentioned the name of the REC/IRB, and 95% (19/20) of the studies also clearly indicated the ethics review number, while one study, from China [21] published in Chinese Medicine did not specify the ethics review number, which is actually not in accordance with the requirements of the journal. In Q2 section, 6.25% (3/48) manuscripts did not mention any information of REC/IRB and ethics review number. Of the remaining 45 studies, 3 studies published in Complementary Therapies in Clinical Practice and 1 study published in COMPLEMENTARY THERAPIES IN MEDICINE only had the name of the ethics review committee, which was not requested by either journal, and 2 studies published in INTEGRATIVE CANCER THERAPIES and 3 studies published in BMC Complementary Medicine and Therapies only had the name of the ethics review committee without mentioning the ethics review number either, although both journals made clear requests for the provision of the ethics review number.
As for obtaining IC forms from patients, it was obtained from study subjects in all 68 studies, 95% (19/20) studies in Q1 section and 72.92% (35/48) studies in Q2 section mentioned that it was signing an IC form, the others were unknown.
Of all 68 manuscripts, only 4 manuscripts in Q1 section mentioned compliance with the DoH, while 28 manuscripts in Q2 section mentioned the DoH. Beyond that, there was no reference to other internationally recognized guiding principles mentioned.
https://news.google.com/rss/articles/CBMiSmh0dHBzOi8vYm1jbWVkZXRoaWNzLmJpb21lZGNlbnRyYWwuY29tL2FydGljbGVzLzEwLjExODYvczEyOTEwLTAyNC0wMTA3Ny0x0gEA?oc=5
2024-07-13 07:23:35Z
CBMiSmh0dHBzOi8vYm1jbWVkZXRoaWNzLmJpb21lZGNlbnRyYWwuY29tL2FydGljbGVzLzEwLjExODYvczEyOTEwLTAyNC0wMTA3Ny0x0gEA
Tidak ada komentar:
Posting Komentar